Abstract:
Vitamin B7 (more often referred to as biotin) is a water-soluble B vitamin that plays a crucial role in cellular processes, including fatty acid synthesis, amino acid metabolism, and gluconeogenesis. To maintain a healthy biotin status, humans rely on both dietary intake and gut microbiome vitamin synthesis. A genomic study conducted in 2015 analysed 256 human gut microbial genomes and revealed that only ~40% possess the complete pathway for biotin synthesis. Key producers include Bacteroides fragilis, Prevotella copri, Fusobacterium varium, and Escherichia coli. However, other bacteria, such as Bifidobacterium and Lactobacillus, not only consume biotin but also compete for it. The microbiome composition is determined by the producer-to-consumer ratio, which, along with other factors (such as diet), influences the biotin availability. Emerging research has suggested the link between the imbalanced composition of the microbiome and numerous neurological conditions, as well as obesity and inflammation. Studies in animals confirm the gut microbiome contribution to hosts' overall biotin status, but the precise quantitative contribution in humans is still unknown. Further quantitative research on microbial biotin absorption is crucial for the development of appropriate, evidence-based nutrition strategies to optimise human health.
Introduction:
Biotin is an essential B-vitamin which was recognized over a century ago and proven to be vital for all animals, playing a role as a cofactor for critical metabolic enzymes1. Also known as Vitamin B7, or Vitamin H, it is an essential cofactor for enzymes (carboxylases) that regulate carboxylation and decarboxylation reactions, upon covalent attachment to biotin carboxyl carrier protein playing an important role in gluconeogenesis, fatty acid metabolism, and amino acid metabolism2,10. Mammals like humans cannot synthesize biotin, but it is present in many foods and is produced by gut bacteria, which in combination maintains a healthy level of biotin in humans. Key dietary sources include eggs, liver, dairy, nuts, and leafy vegetables12. Absorption of free biotin occurs primarily in the small intestine via the sodium-dependent multivitamin transporter (SMVT). It is a high-affinity transporter that also mediates uptake of pantothenic acid (vitamin B5) and lipoic acid. Notably, SMVT is expressed not only in the proximal small intestine but also (to a lesser extent) in the colon. This means that biotin produced by colonic bacteria can be absorbed in the large intestine, although less efficiently than in the small intestine4.
A large study conducted in 2015 showed that in gut microbiota, 40% of the bacteria can synthesise biotin, while the remaining percentage rely on biotin uptake3. Taking into consideration that the human gut microbiota has been shown to contain bacteria that synthesize biotin, and bacteria that consume biotin, the shift in the microbiome composition has the potential to influence human health5. Recent findings suggest that certain gut bacteria, such as Bacteroides fragilis, Fusobacterium varium, and Campylobacter coli possess genes involved in biotin biosynthesis4. Conversely, some bacteria, such as Lactobacillus, actively consume biotin, and therefore have specialised genes (such as bioN, bioY, bioM) involved in the process of obtaining the nutrient from the environment6.
Biotin's physiological importance is underscored by the symptoms that emerge upon biotin deficiency. Biotin deficiency in humans is relatively uncommon under normal dietary conditions, thanks to its wide availability in foods12. Biotin deficiency might increase the risk of skin rash, hair loss and nervous system damage, given its crucial role in the carboxyl transfer reactions11. Neurological symptoms can also occur, ranging from depression, lethargy, and numbness to hypotonia and developmental delays in infants4.
16S RNA sequencing has become a standard method to characterize the gut microbiome's composition and its functional potential. By applying 16S rRNA sequencing to gut samples, researchers can detect and quantify the bacteria present, including those known to produce biotin. This approach has been widely used to explore the link between gut microbiota composition and B-vitamin production5. In 2011, Arumugam et al. conducted a metagenomic study, investigating the gut microbiome composition in humans across different populations. The main findings of this study highlight that certain bacterium in human gut microbiome is enriched in biosynthesis of different vitamins, including biotin7. Further exploring and building up on these findings, more recent studies have focused on identifying specific biotin-producing populations in the gut and examining how their presence correlates with humans' (hosts') biotin status4.
This review will summarise key information on biotin-synthesizing bacteria in the gut, its detection using 16S RNA sequencing, including the methodology and limitations. We will also highlight the relationship between biotin-producing bacteria and human health, with future implications in microbiome-based diagnostics.
Biotin structure and synthesis pathway:
Biotin is a complex organic molecule, with the unique fused ring structure and its molecular formula is C10H16N2O3S22. Sulphur-containing tetrahydrothiophene ring is fused to a ureido ring, containing 2 nitrogen atoms, and a valeric acid side chain containing 5 carbons22,23.
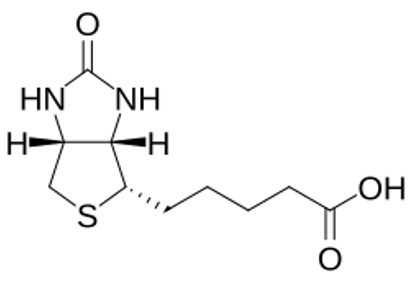
Due to its complex structure, biotin biosynthesis pathway is a complex, energy-consuming pathway that assembles the vitamin from simpler precursors29. The synthesis pathway can be divided into two phases: first is the synthesis of a pimelate-thioester precursor and, second, assembly of the fused bicyclic ring of biotin from that precursor20, 21.
Figure below summarises two steps of biotin synthesis, showing different pathways in gram-positive, gram-negative bacteria, as well as recently discovered pathway in certain species such as Agrobacterium tumefaciens, Brucella melitensis, and Sinorhizobium fredi:
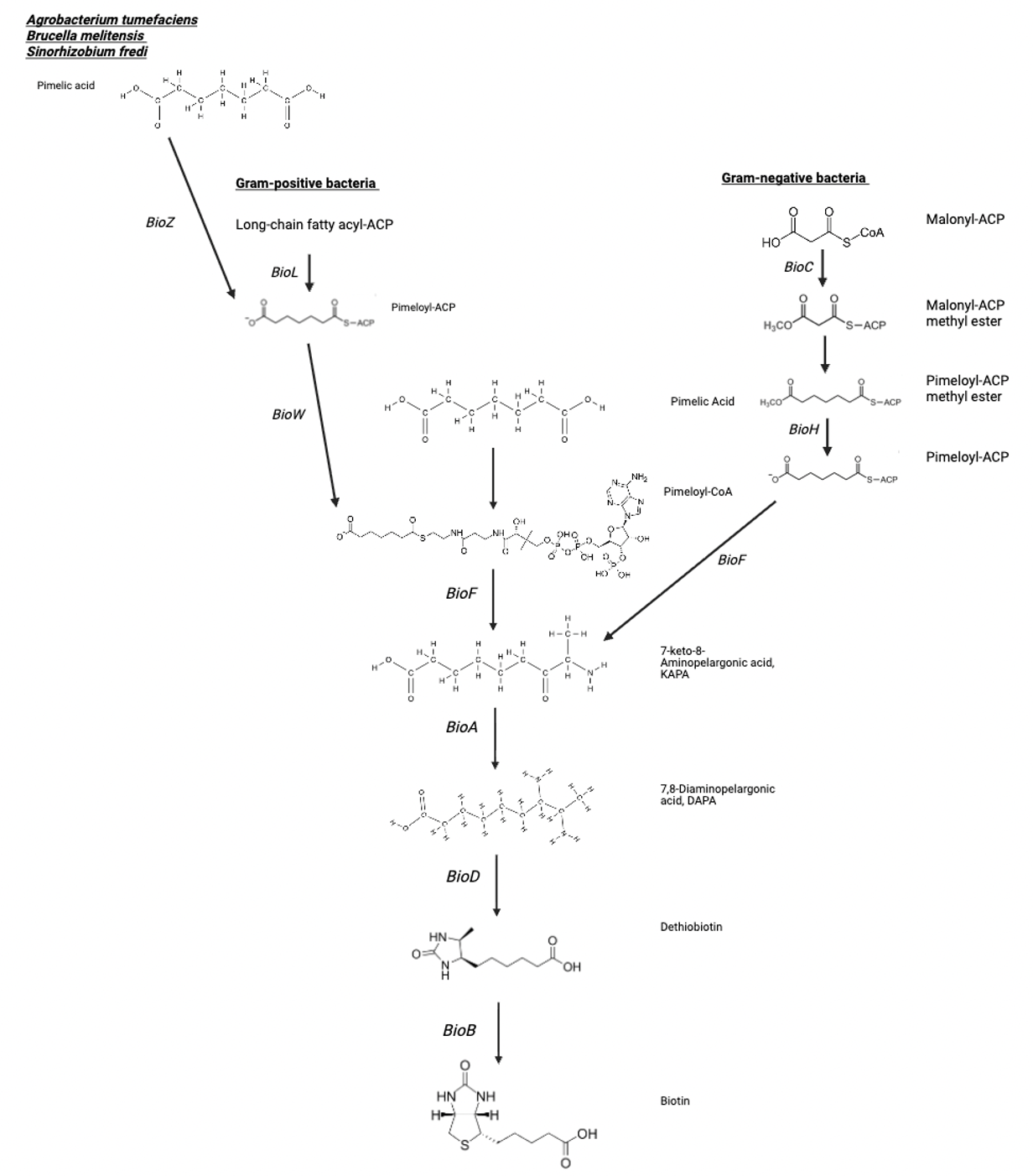
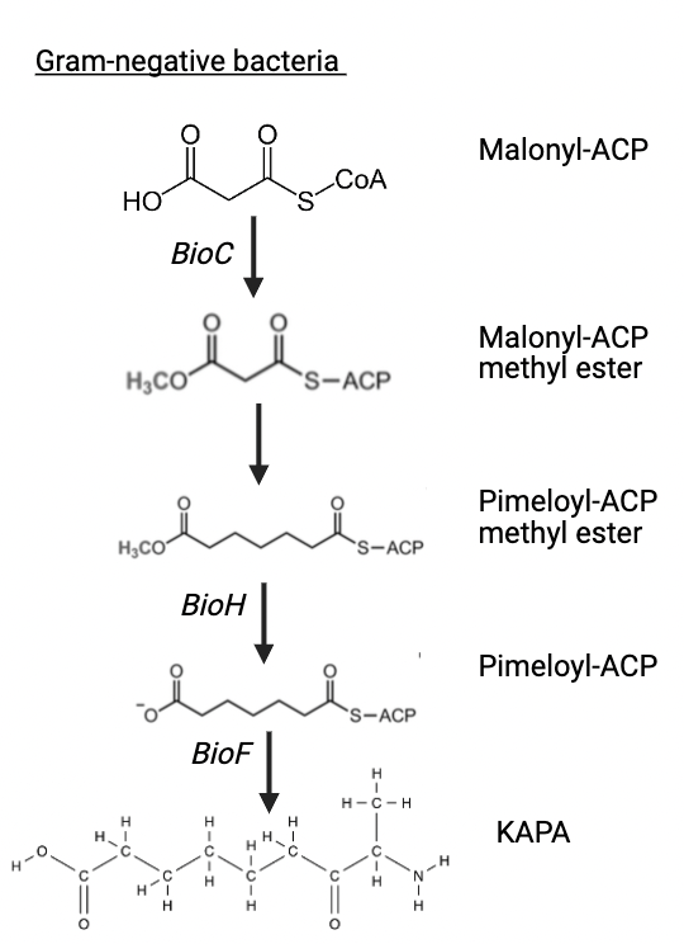
The first phase starts with the bacteria producing a pimeloyl-CoA or pimeloyl-acyl carrier protein (pimeloyl-ACP) moiety, which provides the seven-carbon backbone of biotin's structure20. Different bacterial species generate pimelate-thioester precursor differently, but generally pimelate moiety of biotin is made by a modified fatty acid synthesis pathway24.
In Escherichia coli and other Proteobacteria the pathway involves the sequential action of BioC (malonyl-CoA methyltransferase), and BioH (methylesterase). BioC modifies malonyl-CoA to disguise it as a fatty acid intermediate, enabling its elongation by the fatty acid synthase complex. BioH then removes the methyl group to yield pimeloyl-ACP, the substrate for the later biotin-specific enzymes25.
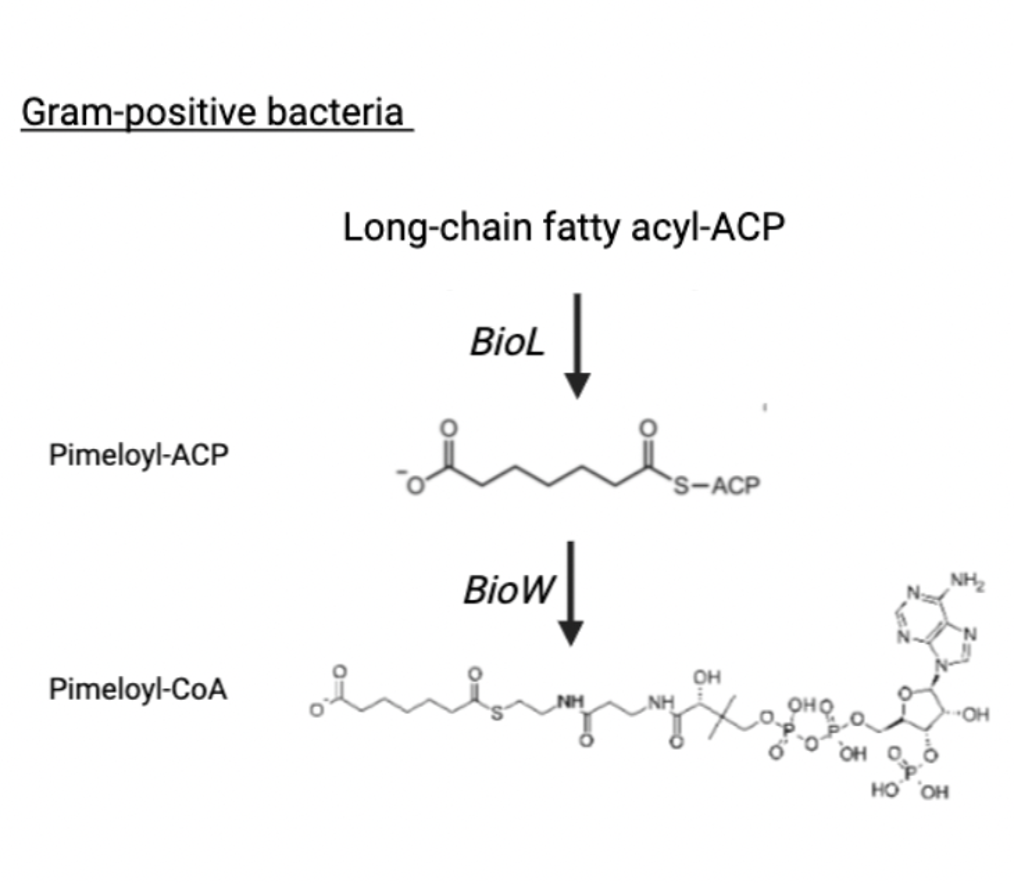
Conversely, Gram-positive bacteria such as Bacillus subtilis use an alternative strategy that bypasses fatty acid synthesis. These organisms utilize BioW (pimelate-CoA ligase), to directly convert free pimelic acid into pimeloyl-CoA26.
And lastly, more recently discovered pathway which can also form the pimelate precursor involves BioZ (pimeloyl-ACP synthetase) and was identified in bacteria such as Agrobacterium tumefaciens, Brucella melitensis, and Sinorhizobium fredi27,28.
Even though different bacteria use different mechanisms, they all form the same product – pimelate-thioester, which then enters second phase of biotin synthesis.
Second stage involves 4 chemical reactions, involving 4 key enzymes: BioF, BioA, BioD, and BioB21. So far, no alternative enzymes have been identified for these reactions, but all 4 are found in all known biotin-producing species. This further highlights the evolutionary conservation among species21. The figure below shows 4 chemical reactions leading to the production of biotin:
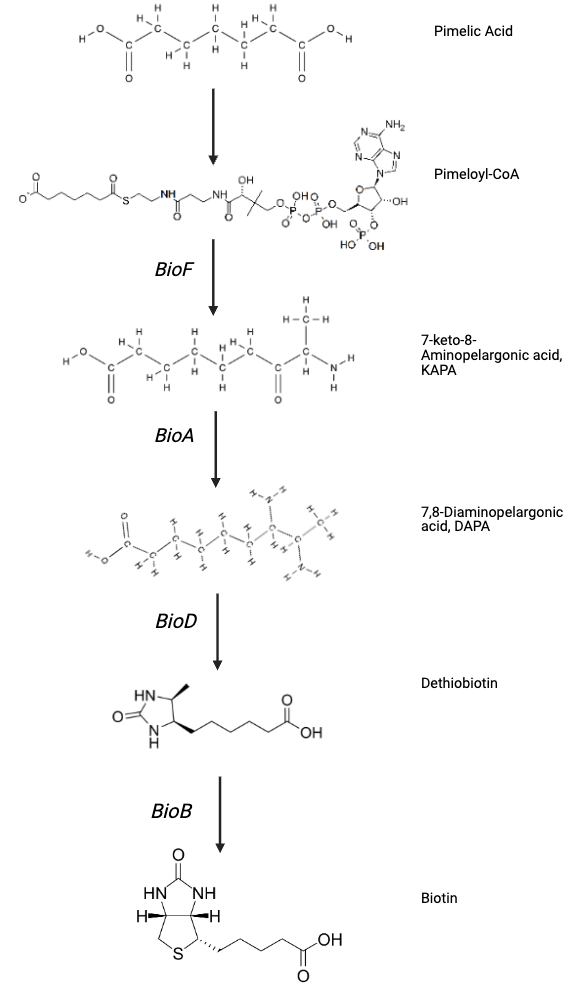
In summary, biotin synthesis is a two-stage pathway, where different bacteria use different pathways to generate pimelate-thioester, followed by generation of biotin bicyclic ring structure using 4 different enzymes30. These enzymes are encoded by genes clustered in bio-operon, whose presence is closely associated with the bacteria's ability to synthesise biotin de novo.
Biotin-producing bacteria
Before 2015, it was largely assumed that gut bacteria contributed meaningfully to biotin availability in humans, however the theoretical link was not clearly demonstrated in any experiments. Early studies had suggested that certain colonic bacteria, such as Bacteroides fragilis and E. coli, could synthesize biotin in vitro, and germ-free animal models showed reduced B-vitamin levels compared to conventionally raised controls. However, these findings lacked clarity about the actual contribution to host biotin status due to the lack of metagenomic tools or large-scale genomic databases, and the idea of gut-derived biotin remained theoretical and not experimentally verified.
Recently it has been proven that healthy human gut microbiome usually characterised by a presence of both biotin-producing and biotin-consuming (auxotrophs) bacteria9. Groundbreaking genomic study conducted in 2015 analysed 256 human gut microbial genomes for vitamin pathways and identified that approximately only 40% possess the complete pathway for the biotin synthesis, however the distribution is uneven across different bacterial groups3.
| Biotin-producing bacteria | Biotin-consuming bacteria |
|---|---|
|
Bacteroides fragilis Prevotella copri (Bacteroidetes) Fusobacterium varium (Fusobacteria) Escherichia Coli Campylobacter coli (Proteobacteria) |
Bifidobacterium adolescentis Bifidobacterium breve Bifidobacterium longum Ruminococcus Lactobacillus murinus Lactobacillus Helveticus Prevotella |
One of the primary biotin producers are members of Bacteroidetes, especially the genus Bacteroides, with Bacteroides fragilis being a prominent example of a biotin-producing gut bacterium4,8. Another example is Prevotella copri, which has a full bio-operon, meaning all the genes required for the biotin synthesis8. Microbiomes dominated by Bacteroides show high levels of biotin biosynthesis genes, therefore further supporting the correlation between Bacteroides-enriched microbiomes and high biotin biosynthesis capacity5,7.
Detection methodology
To identify biotin-producing bacteria 16S rRNA gene sequencing technique is employed. The 16S rRNA gene is present in all bacteria and contains hypervariable regions that provide a "fingerprint" for different taxa. By amplifying and sequencing this gene from DNA extracted from stool samples, researchers can determine the relative abundance of bacterial genera and species present in the gut13.
Tools like PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) take 16S data and computationally predict metagenomic function by mapping known gene content of reference genomes onto the community profile5. Many studies support the accuracy and efficiency of PICRUSt while handling thousands of references genomes34.
Research Applications
Modern scientific understanding establishes human gut microbiota as a vital non-dietary source of biotin, further implicated in a range of health conditions beyond the gastrointestinal disorders18. For example, emerging studies on gut-brain axis revealed substantial evidence that suggest significant role of gut microbiome in neurological disorders, such as Alzheimer's disease, Parkinsons disease and multiple sclerosis16,17.
Another finding took place in 2022, where Belda et al. combined metagenomics, metatranscriptomics, and metabolomics in a large human cohort to investigate correlation between biotin and obesity5. Main findings highlight that those individuals with severe obesity had fewer biotin-producing taxa, lower expression of bio genes and significantly reduced levels of circulating5. Interestingly, after interventions, such as bariatric surgery, gut microbiome biotin synthesising potential increased, improving overall biotin levels5. This means that normalising circulating biotin levels could help prevent the deterioration of metabolic states in severe obesity5.
Animal studies
Animal studies provide crucial foundational evidence for the gut microbiome's contribution to host biotin status. A study by Hayashi et al. (2017) demonstrated that biotin deficiency in mice led to the overgrowth of Lactobacillus murinus, a biotin auxotroph. This led to the onset of alopecia, suggesting biotin's role in skin and hair health, providing a new treatment angle, although more evidence is needed35. A study by Belda et al. in 2022 utilized germ-free animals, which lack an entire microbiota, as well as antibiotic-treated animals, with significantly depleted microbiota, consistently confirming a substantial microbial contribution to host biotin levels5. The absence or depletion of the gut microbiota in these models directly caused altered biotin levels in the host, further strengthening the link between the gut microbiome and systemic biotin levels5. The same study showed that human-to-mice microbiota transfer experiments directly influenced the level of biotin in the recipient mouse's blood. This in vivo experiment can provide a good scientific foundation for future techniques involving the targeting of the gut microbiome to influence biotin status.
Research Limitations
Despite significant advances in gut microbiome research, there are limitations to the current understanding of the biotin production in the gut and its implications on human health. Most studies on gut microbiome's contribution to hosts biotin status have been conducted in animal models, waiting for future direct causal evidence in humans. Moreover, factors such as absorption rate, transporter expression variation amongst individuals must be considered when quantifying vitamin production. Future research should aim to expand reference databases, as well as conducting clinical trials on humans to account for variability in samples.
Conclusion
In the past 10 years the research has established human gut microbiome as an indispensable source of non-dietary biotin. Microbial community has been experimentally proven to possess the capacity for de novo synthesis of biotin, significantly contributing to the host's overall biotin status. Key bacterial species, such as Bacteroides fragilis and Prevotella copri, are recognized as primary producers, characterising the interplay between biotin-producing and biotin-consuming microbes. This review article summarised the findings and discoveries happened in the past 10 years, increasing our confidence levels regarding the relationship between human gut microbiome and hosts biotin levels. After reviewing all the information, we can confidently state that there is scientific evidence that several human gut bacteria can produce biotin and moreover contribute to hosts biotin levels.
Despite significant progress in understanding the mechanisms and identifying key microbial players, the precise quantification of the absorbed amount of microbially-derived biotin in humans remains unknown due to the lack of experimental evidence. Therefore, there is an urgent need for clinical trials in humans to establish the extent of gut microbiome contribution.
